Let me first set the stage for you by talking about two of my least favorite things on the entire planet: mosquitoes and viruses.
 |
| Female Culiseta longiareolata (via Wikipedia) |
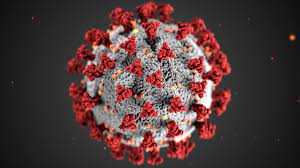 |
| Coronavirus illustration (via FDA.gov) |
Doesn't just looking at them give you the heebie-jeebies!? The mosquito's creepy proboscis (its "straw") and the ominous feeling that even just staring at a virus gives you are bad enough, but what if I told you that there was a biological entity that combined these two oh-so-wonderful things? Allow me to introduce to you: the bacteriophage!
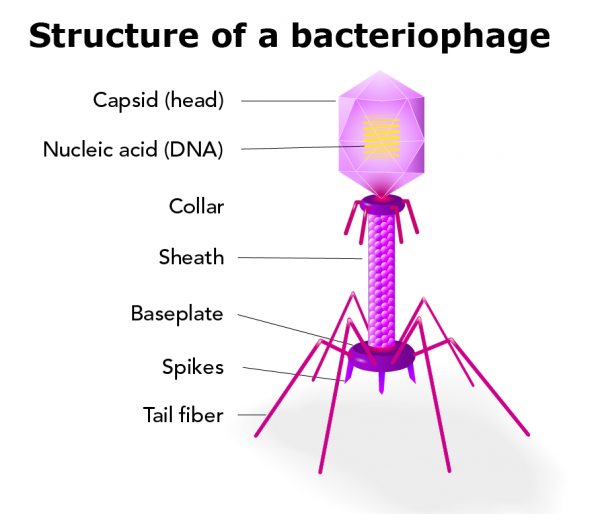 |
| Bacteriophage structure (via istockphoto) |
Measuring in at a whopping 24 - 200 nm (that's about 1/500th the width of a human hair), the bacteriophage is a special type of virus composed of genetic information (either DNA or RNA) encased by a protein shell, complete with a rather eerie-looking tailpiece, and some even have filaments! Bacteriophages, or as I like to call them "the phage" (it is true, though, that they are often referred to simply as "phages"), are some of the most abundant biological entities to exist on the planet, with there being roughly ten phages to exist per host cell (give or take). There’s about five million trillion trillion bacteria on our planet (that’s 5 ✕10³⁰, or 5 and thirty zeroes -- approximately 667 billion times more than the number of grains of sand on Earth); take that number and multiply it by ten, and that’s how many phages we’ve got hanging all around us!
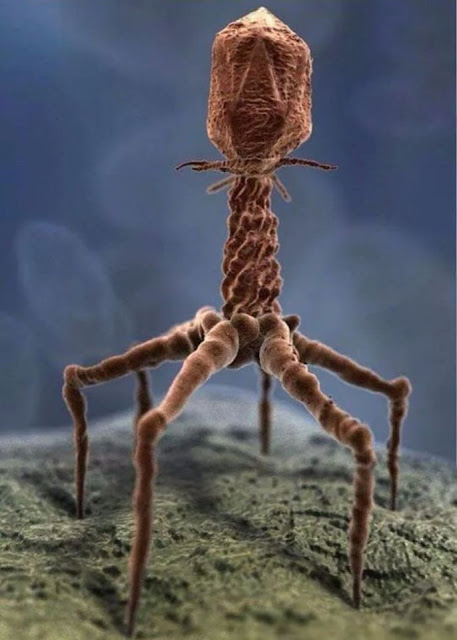 |
| Scientific animation of a T4 bacteriophage (via XVIVO) |
Now, before I get too carried away talking about these creepy little phages, I think we should take a moment to analyze what the word “bacteriophage” actually means. As you can probably guess, “bacterio” stems from the word “bacteria” (you know, those little prokaryotic organisms that are just about everywhere and are honestly pretty darn cool?), but “phage”, which might be a little more foreign to you, originates from the Greek word “phagein”, which means “to eat”; that means that bacteriophages “eat” bacteria! (If you’re interested in reading a bit more about the etymology of the bacteriophage, have a little looksie here!) Now, all this to say that bacteriophages are harmless to people and do NOT affect us! This means that you can keep on reading this blog post without the lingering worry in the back of your mind that phages are going to take over the world one day! With that thought out of your mind (as I’m sure it was), let’s get into some of the nitty-gritty science of these impressive little viruses:
 |
| Bacteriophages infecting a cell (via shutterstock) |
As I mentioned earlier, bacteriophages are a specific type of virus. To reproduce and infect more hosts, as viruses do, the phage inject their genetic information into their target cells (which are all bacteria), and undergo either a lytic or lysogenic reproductive cycle. While I won’t go into too much detail about these two processes (although you’re welcome to read about them here), the gist of it is that a lytic cycle results in the violent death (via explosion) of the bacterial host cell, while the lysogenic cycle allows for a more silent reproductive path that enables the proliferation of the virus without impacting the host cell too much; this is accomplished through integration of the virus’ DNA into the host cell’s genome (though, after a certain point a lysogenic cycle often turns into a lytic cycle and the bacterium bursts). The purpose of these reproductive cycles is to force the bacterium to synthesize parts of the virus that can later assemble into new, full-fledged bacteriophages that can then go on to infect more cells. This happens because the most basic goal of all life on Earth (and life-akin entities such as viruses) is to reproduce. Since viruses can’t reproduce on their own, they need other cells to produce the proper components of a virus so that they can continue infecting other cells. The phage accomplish this task through the passage of their genetic information stored in either DNA or RNA to a host bacterium, which then undergoes translation (or transcription and translation if the phage contained DNA), and finally the host cell produces a protein. Once enough proteins are made, they accumulate at the cell membrane and assemble into new bacteriophages, then exit the bacterium once the cell bursts.
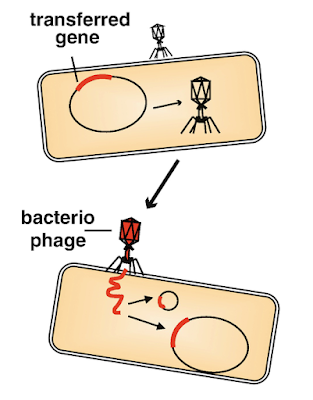 |
| Transduction (via springernature.com) |
This hijacking of bacterial machinery often results in a form of horizontal gene transfer called transduction. Normally when we think about the passage of genetic information, we think of vertical gene transfer -- that’s the normal (and rather boring) story of how genes are passed down “when a mommy loves a daddy”, but there’s actually a completely different and fairly common method of gene transfer that accounts for up to one-third of a bacterium’s genome. This is what is known as horizontal gene transfer. Horizontal gene transfer occurs in four primary ways, but I’m only going to focus on transduction in this post (although, if you’re just super interested in horizontal gene transfer and want a quick overview, this Khan Academy article is for you!). At its most basic level, horizontal gene transfer is defined as “the non-sexual movement of genetic information between genomes”, meaning that the forced uptake of DNA by bacteria can, in fact, be a form of gene transfer even though the genetic material did not come from parent cells. In transduction, genetic information from one bacterium is transferred to another bacterium by a virus. Oftentimes, this transfer is accidental and happens when a bacteriophage contains some genes from a host cell that got packaged into its capsid head by mistake. These genes are then implanted into the phage’s next victim after it injects its virulent genetic information into a new host. When the foreign DNA is integrated into the host cell’s genome, the DNA from the first bacterium becomes part of the new host cell’s genome, and thus the host’s genome is different than it was before. It’s kind of crazy to think that these little alien-looking viruses are a huge source of genetic variation in our world, especially by accident, but they are!
 |
| Antibiotics (via flickr) |
Now, as interesting as I think bacteriophages are, I’ve recently been informed that most people don’t find learning about viruses, and their structures, and their purposes, and their methods of gene transfer to be the most enthralling thing ever. So, I figure it’s about time I talk about why these little suckers are so important to us as people. Prior to the discovery of the first antibiotic in 1910, and the later groundbreaking discovery of penicillin in 1928, humans were subjected to bacterial infections that could render us very dead, very quickly. Many of these same infections, however, are now easily treatable with antibiotics -- or at least they were. As overuse of antibiotics for minor infections has become more prominent, and as bacteria evolve to resist our antibiotic treatments, superbugs (which are bacteria “resistant to most of the antibiotics … commonly used to treat the infections they cause”) and the onset of an antimicrobial resistance crisis have become of great concern to our society. One solution that scientists have turned to in order to combat the growing issue of widespread antibiotic resistance is phage therapy. In phage therapy, specially engineered bacteriophages target specific bacterial cells and obliterate them, thus clearing them from the human body. This is beneficial for many reasons, namely that the phage do not target human cells, they do not need to be constantly injected into patients, all bacteria are known to lyse from some sort of phage, and, since they’re naturally occurring, bacteriophages can evolve right alongside the ever-changing bacteria, allowing them to remain effective for longer periods of time. Aside from the biological pluses of phage therapy, this treatment has also been shown to reduce the cost that patients must pay for life-saving treatments. (If you’re interested in learning about phage therapy a bit more in-depth, I HIGHLY recommend watching the above video!)
 |
Two scientists in a bacteria phages lab. Photograph: Aimee Obidzinski. https://www.theguardian.com/science/2022/may/13/phage-therapy-fight-against-drug-resistant-infections-antibiotics |
There are, unfortunately, some barriers that make phage therapy a less viable treatment option than, say, antibiotics. Many of the issues with phage therapy arise from the fact that it has not been studied in depth by most countries, so its long-term effects and practicality are largely unknown. Here’s what we know so far, though: while phages being specific to bacteria is a benefit since they protect human cells from unnecessary damage, this also means that it’s harder to find bacteriophages that fight specific infections since they have to be able to perfectly match with a given bacteria. This causes a delay in phage selection, and can make it more difficult to target a specific type of bacteria. The issue that's been most concerning during the course of phage therapy research is that, since they are viruses and do possess many characteristics of life, bacteriophages can evolve and change during the time they are being studied, administered, or even while they’re inside a person, posing possible risks to the study that could jeopardize the future of phage therapy. There are, however, numerous studies being conducted that seek to investigate the best way to further pursue phage therapy and determine the safest, most effective way to administer the phages to humans. A series of experiments conducted in Paris earlier this year explored many of the lesser-understood factors of phage therapy, including the impact of how the viruses are put into patients, as well as details about the viruses themselves (ie: how quickly they infect bacteria, grow, and complete the lytic cycle). The findings of the study are promising, and open the door to quicker, more in-depth, and less expensive research into phage therapy.
So, to recap: bacteriophages are viruses; phages increase genetic variation among bacteria; the phage do not harm humans, and can instead help us combat antibiotic resistance.Now, that was a whoollllleeee lot of reading, so, as a reward, I’m now going to grace you with a couple of my favorite bacteriophage memes that I found over the course of my research (and before you ask: yes, phage memes constituted approximately 25% of the time I spent writing this blog post!).
So, to recap: bacteriophages are viruses; phages increase genetic variation among bacteria; the phage do not harm humans, and can instead help us combat antibiotic resistance.Now, that was a whoollllleeee lot of reading, so, as a reward, I’m now going to grace you with a couple of my favorite bacteriophage memes that I found over the course of my research (and before you ask: yes, phage memes constituted approximately 25% of the time I spent writing this blog post!).
 |
| Phage meme (via Facebook) |
 |
| (Nicolas) Cage on the Phage (via ASAP Science) |
My final wish for you, my dear reader, is that you’re leaving this little biology rambling of mine with a love for bacteriophages just as strong as mine. I adore these strange, alien-esque, non-living entities so much so that I have set out to crochet one of my own. I’m not very far into it, but I’ve got the capsid head done, which I’ve attached here for your viewing pleasure. I bid you a final farewell, my fellow science lover!
 |
| Crochet capsid head |
This was a very informative post and you're a very good writer! Considering that bacteriophages are "some of the most abundant biological entities to exist on the planet", I really haven't known a lot about them (I only heard about them in bio class), and now I know so much more about them. Thinking about how they can destroy bacteria at first, I was a little concerned for humans, but it never occurred to me they could actually be used to fight off harmful infections, as bacteria are coming more immune to antibiotics. Awesome job! I liked the memes at the end as well :)
ReplyDeleteYay Cassidy!!! Yes I already read this but it is awesome. I love your passion for the creepy bacteriophage. You have infected me (lol) so much that one day for Wordle I almost guessed "phage" before "phase" (the word was phase). Considering microbiology was always my weak point I really find this post fascinating! Despite looking like aliens bacteriophages are so important to our life on Earth and I do think the way they work is super unique, and terrifying. Like, imagine if there was an alien species that could inject its DNA into us. It's like mind control. You should make a movie about that.
ReplyDelete